Coupling between Lung and Metabolism
⇰ Science Topic information page
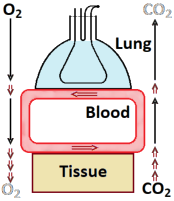 The lung transports oxygen (O2) from outside to the
alveolar space
and carbon dioxide (CO2) the other way around. This is by a
convective process
since it implies actual gas flow. That is not possible
between lung air and blood; here, it is by diffusion. Diffusion is very
effective on short distances but completely inadequate over longer ones;
as explained in this page. So, between lung
and the various tissues, blood has to flow carrying O2 and CO2
to and from – convection. No flow within tissues, so diffusive transport
there.
The lung transports oxygen (O2) from outside to the
alveolar space
and carbon dioxide (CO2) the other way around. This is by a
convective process
since it implies actual gas flow. That is not possible
between lung air and blood; here, it is by diffusion. Diffusion is very
effective on short distances but completely inadequate over longer ones;
as explained in this page. So, between lung
and the various tissues, blood has to flow carrying O2 and CO2
to and from – convection. No flow within tissues, so diffusive transport
there.
The figure is extremely simplified but is for an overall idea and as a basis
for modelling. Most important: it takes some time between lung and tissues.
A blood flow of 5 L/min and a blood volume of 5 L implies a 1 minute
cycle time, so, on average half a minute delay. This makes coupling between
metabolism
and ventilation slow, and has consequences for the regulations:
these would become unstable if too fast.
It complicates modelling of lung gas values for changing metabolism, e.g.,
when someone starts heavy work. Note, that this half minute is an average –
for tissue distant from the lung, as leg muscle, it will even be more. So, the
delay even is heterogeneous too: some CO2 arrives after a couple of seconds,
some after a minute.
Again, modelling is through the gas law. In both the blood-lung and the
blood-tissue transfer, all CO2 produced in the tissue has to be
exchanged leading to:
|
PTot˙VCO2 =
˙nCO2RT =
Q (cv,CO2 − ca,CO2)RT
|
where Q is the blood flow, c concentration and the indices a, v refer to arterial
– from lung to tissue – and venous – from tissue to lung –
blood respectively. There is a direct relation (1)
between cCO2 and
PCO2 in the blood, the CO2 Buffer Curve; the c
determines the amount in the blood, the P the driving force over the boundary.
CO2 is very well buffered in the blood; the total amount c is much
larger than would be expected only from the partial pressure.
Coming from the lung, PCO2=5.3 kPa; after passing the tissues,
6 kPa. This means that an increasing tissue production only slowly raises
PCO2.
The main concern of ventilation regulation is on CO2; O2
mostly is no problem. Transfer modelling is the same as for CO2:
|
PTot˙VO2 =
˙nO2RT =
Q cHb(Sa,CO2 − Sv,CO2)RT
|
where cHb is oxygen binding concentration in the blood –
caveat: four times the concentration of the binding molecule hemoglobin (Hb)
but yet often denoted 'hemoglobin concentration' – and S
saturation (2) of the Hb.
(1) The reaction between CO2 and
its main buffer HCO3−
is slow but considerably speeded up by carbonic anhydrase in the red blood cell.
(2) Relative amount of O2 bound,
here, as a fraction instead of percentage.
Back to the top of the topic
–
 The lung transports oxygen (O2) from outside to the
alveolar space
and carbon dioxide (CO2) the other way around. This is by a
convective process
since it implies actual gas flow. That is not possible
between lung air and blood; here, it is by diffusion. Diffusion is very
effective on short distances but completely inadequate over longer ones;
as explained in this page. So, between lung
and the various tissues, blood has to flow carrying O2 and CO2
to and from – convection. No flow within tissues, so diffusive transport
there.
The lung transports oxygen (O2) from outside to the
alveolar space
and carbon dioxide (CO2) the other way around. This is by a
convective process
since it implies actual gas flow. That is not possible
between lung air and blood; here, it is by diffusion. Diffusion is very
effective on short distances but completely inadequate over longer ones;
as explained in this page. So, between lung
and the various tissues, blood has to flow carrying O2 and CO2
to and from – convection. No flow within tissues, so diffusive transport
there.