Basic Ventilation Model
⇰ Science Topic information page
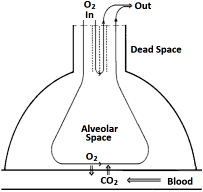 The ventilatory function of the lung can be explained already in a very
simplified (1) model. Oxygen (O2)
has to be transported from the outside
air to the blood, and carbon dioxide (CO2) the other way, thereby
passing airways and alveoli.
The latter are the locations where gases are exchanged with the blood, and are taken
together as Alveolar Space (VA), the remaining part is (physiological)
Dead Space (VD). The conditions are, fixed values of O2
and CO2 (2)
partial pressure
in the outside air, PO2,in and PCO2,in respectively, and
fixed values of oxygen uptake (˙VO2)
and carbon CO2 production
(˙VCO2) of the body.
The ventilatory function of the lung can be explained already in a very
simplified (1) model. Oxygen (O2)
has to be transported from the outside
air to the blood, and carbon dioxide (CO2) the other way, thereby
passing airways and alveoli.
The latter are the locations where gases are exchanged with the blood, and are taken
together as Alveolar Space (VA), the remaining part is (physiological)
Dead Space (VD). The conditions are, fixed values of O2
and CO2 (2)
partial pressure
in the outside air, PO2,in and PCO2,in respectively, and
fixed values of oxygen uptake (˙VO2)
and carbon CO2 production
(˙VCO2) of the body.
Breathing is, by intaking and exhaling a Tidal Volume (VT)
at a frequency fR mostly measured in per minute. Then, the process is simple
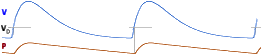
and can be explained without mathematics. For oxygen, this is show in the figure below.
The inhaled air does not reach the alveolar space instantly but first has to pass through
the dead space. Then, oxygen pressure P is increased until inspiration ends, after
which it decreases due to uptake by the blood. The amounts must be equal, but below that
will be explained for CO2 since it is easier.
Modelling gas transport is based on the gas law PV=nRT but since RT is a constant
in the body, it is done based on P×V being the "amount of gas".
As to a gas mixture, there are two equivalent possibilities:
– PTotVX – Total gas pressure times
relative volume of the gas X
– PXVTot – Partial pressure of gas X
times total volume
Then, for CO2 – of course the formulas are also for volume per time:
|
PTot˙VCO2 =
PE,CO2 fR (VT − VD)
|
where the End-Expiratory CO2 Pressure PE,CO2 is
what was in the lung. This formula is used to determine VD.
But there is a considerable pitfall in calculations with gases, due to the fact
that circumstances can be very different. In physics, dry gases at 0°C are
considered, which is indicated by STPD (Standard Temperature and
Pressure, Dry), in lung function body conditions BTPS
(Body Temperature and Pressure, Saturated with
water vapour), 37°C. Standard Pressure is 101.3 kPa but in the body 6.3 kPa
is 'taken away' by the water vapour so that only 95 kPa remains for the
gases. Simple math then learns, that the difference is considerable, about 20%.
(1) In particular in patients, the lung can be
much more complicated.
(2) At sea level, there is about 20% O2
but only 0.04% CO2 so that in physiology PCO2≅0.
Back to the top of the topic
or to outside air
 The ventilatory function of the lung can be explained already in a very
simplified (1) model. Oxygen (O2)
has to be transported from the outside
air to the blood, and carbon dioxide (CO2) the other way, thereby
passing airways and alveoli.
The latter are the locations where gases are exchanged with the blood, and are taken
together as Alveolar Space (VA), the remaining part is (physiological)
Dead Space (VD). The conditions are, fixed values of O2
and CO2 (2)
partial pressure
in the outside air, PO2,in and PCO2,in respectively, and
fixed values of oxygen uptake (˙VO2)
and carbon CO2 production
(˙VCO2) of the body.
The ventilatory function of the lung can be explained already in a very
simplified (1) model. Oxygen (O2)
has to be transported from the outside
air to the blood, and carbon dioxide (CO2) the other way, thereby
passing airways and alveoli.
The latter are the locations where gases are exchanged with the blood, and are taken
together as Alveolar Space (VA), the remaining part is (physiological)
Dead Space (VD). The conditions are, fixed values of O2
and CO2 (2)
partial pressure
in the outside air, PO2,in and PCO2,in respectively, and
fixed values of oxygen uptake (˙VO2)
and carbon CO2 production
(˙VCO2) of the body.
