 Yet, the principles are easy:
Yet, the principles are easy:
– CO binds at the same locations as O2, but very much stronger
– the reaction to remove CO from Hb is very slow
Although binding of carbon monoxide (CO) to hemoglobin (Hb) and its interaction with oxygen (O2) is chemically well understood, its consequences in humans are often misjudged. This is partly due to the fact that common measuring equipment does not "see" the CO bound Hb so that things look fine – but are not!
 Yet, the principles are easy:
Yet, the principles are easy:
– CO binds at the same locations as O2, but very much stronger
– the reaction to remove CO from Hb is very slow
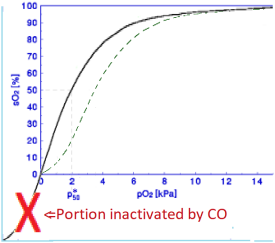
In fact, this leads to an "inactivation" of part of the Hb – see figure – where the remaining part behaves different from the Standard Curve – dashed green line in the figure – in fact, for the body, worse. The program SatCur very nicely shows why. Note, that measurements show normal saturations but those regard only part of the hemoglobin.
The carbon monoxide CANNOT actively been removed from the hemoglobin. It has to come loose by itself – and that is slow – but a high oxygen pressure will prevent it from being bound again by occupying the binding site.
Chemically, it is easy. The binding sites do not "see" whether there is O2 bound or CO. What matters is total amount of reactive species, mostly expressed as PO2+MPCO where PO2, PCO are oxygen and carbon monoxide partial pressure respectively and M is a constant to account for the fact that CO is much more reactive. For human blood, it is about 250.
This principle is true for all binding models and can be worked out accordingly. For the Hill model, it leads to apparent parameters:
| P50* = P50 [ | 1 − FCO | ] | (1-1/n) |
| 1 + FCO |
| n* = n | 1 + FCO |
| 1 + nFCO |